|
|
|
CONTENTS |
|
In the previous section we showed that certain nuclei are not completely
stable and eventually undergo an internal change that will produce a more
stable nuclear structure. This spontaneous change is a radioactive
transition. In some older literature this event is called a nuclear
disintegration. The terminology is misleading because the nucleus does not
disintegrate; it simply undergoes a slight change. This event is
illustrated in the figure below. The original nucleus is designated the parent,
and the nucleus after the transition is designated the daughter. In
radioactive transitions, energy is emitted as radiation. The types of
radiation encountered in nuclear medicine are shown below. The
radiation is in the form of either energetic particles or photons.
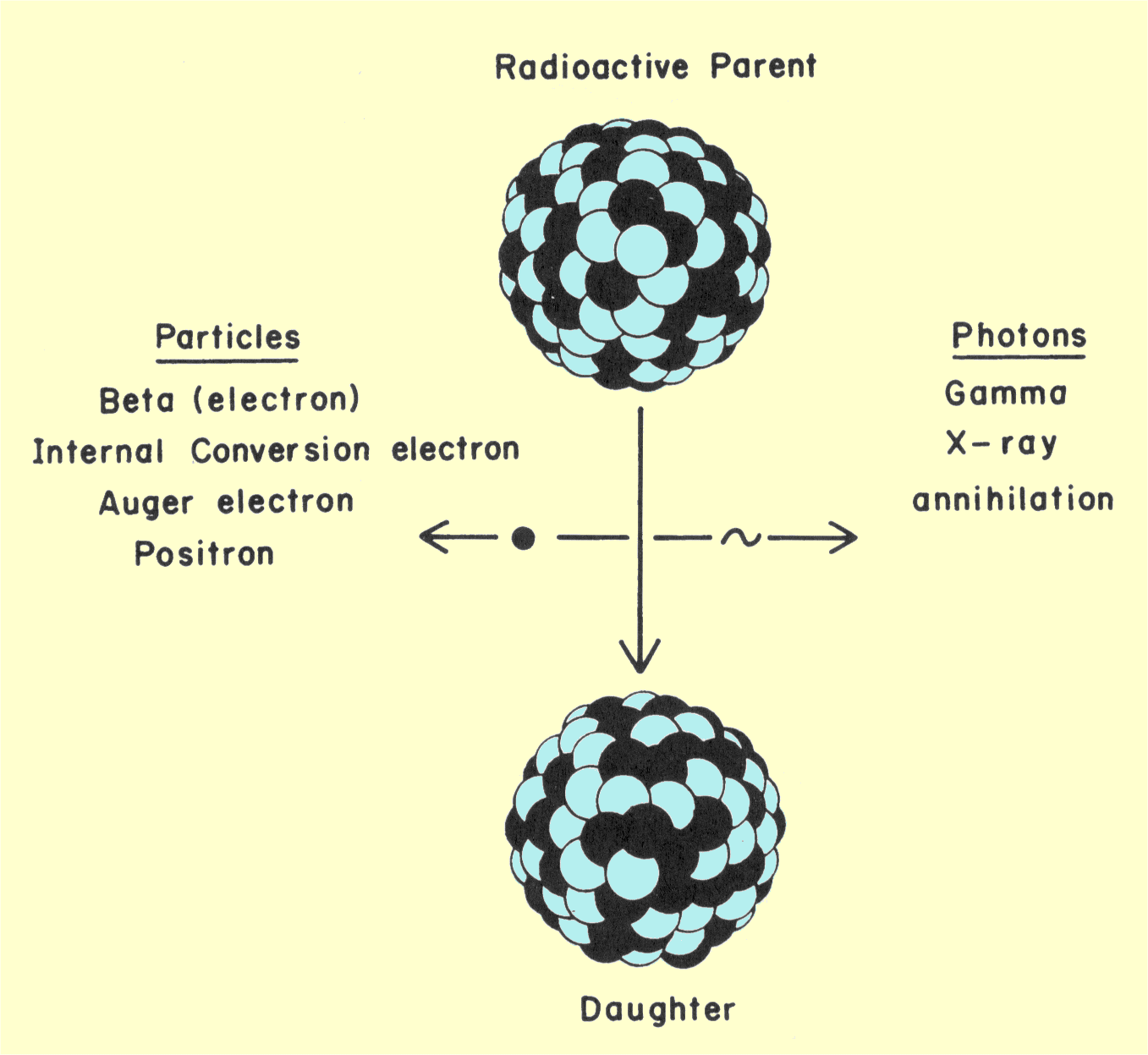
Various Radiations Produced by Radioactive Transitions
Most radionuclides emit a combination of radiations; the
types depend on the physical characteristics of the nucleus and are
considered in more detail later. The daughter nucleus can be either stable
or a radioactive or metastable nucleus that will undergo another
transition in the future.
In most in-vivo nuclear medicine procedures it is desirable
to use a radionuclide that emits photons in the range of 100 to 500 keV.
The penetrating ability of photons is related to their energy. Many
photons in this energy range can emanate from the body, but not penetrate
through the detector and be lost. Particle radiation is not useful in most
diagnostic procedures. In fact, it is usually undesirable because it
deposits its energy in the body close to the site of origin and can
contribute significantly to patient dose without contributing to
diagnostic information. With many radionuclides, particle radiation is a
byproduct of the transition required for desirable photon emissions.
It is often useful to construct a diagram to show changes in
the nucleus and the radiation emitted during a radioactive transition. A
transition diagram, sometimes referred to as a decay scheme, is shown
below. Two types of changes occur within a nucleus: loss of energy
and, possibly, a change in atomic number in an isobaric transition. In the
transition diagram, relative nuclear energy is represented by vertical
distance and relative atomic number by horizontal distance. The actual
scales are not usually shown as they are in the figure below. The position of
the nucleus before and after the transition is represented by horizontal
lines in the diagram. In the figure below the image of a nucleus is resting on
these lines. This is not generally found in the conventional diagram but
is here to help us follow the transition. The steps in the transition are
represented by lines running downward. The transition always moves
downward because the nucleus is decreasing its energy by emitting
radiation. If the atomic number changes (isobaric transition), the
transition line will slant to the right or left.

Diagram Showing Radioactive Transition
The vertical distance between the parent and daughter
positions represents the total transition energy. This value is always
specific for the transition associated with each radioactive nuclide. But
all nuclei of the same nuclide do not necessarily go from the parent to
daughter state in the same way. The significance of this is discussed in
detail later.
|
|
|
|
ISOBARIC TRANSITIONS
|
CONTENTS |
|
Most
radioactive transitions have several steps. For most radionuclides, the
first step is an isobaric transition usually followed by an isomeric
transition and interactions with orbiting electrons. The three types of
isobaric transitions of interest to us are (1) beta emission, (2) positron
emission, and (3) electron capture.
In nuclear stability, the neutron-proton ratio (N/P) is
crucial. If it is too low or too high, the nucleus will eventually
rearrange itself into a more stable configuration. Beta radiation, which
is the emission of energetic electrons, results when an N/P ratio is too
high for stability; positron emission or electron capture occurs when it
is too low for stability. These two conditions are represented by specific
areas of the nuclide chart shown below. Beta emitters are above
the stable nuclides, and positron emitters and electron capture nuclides
are below.
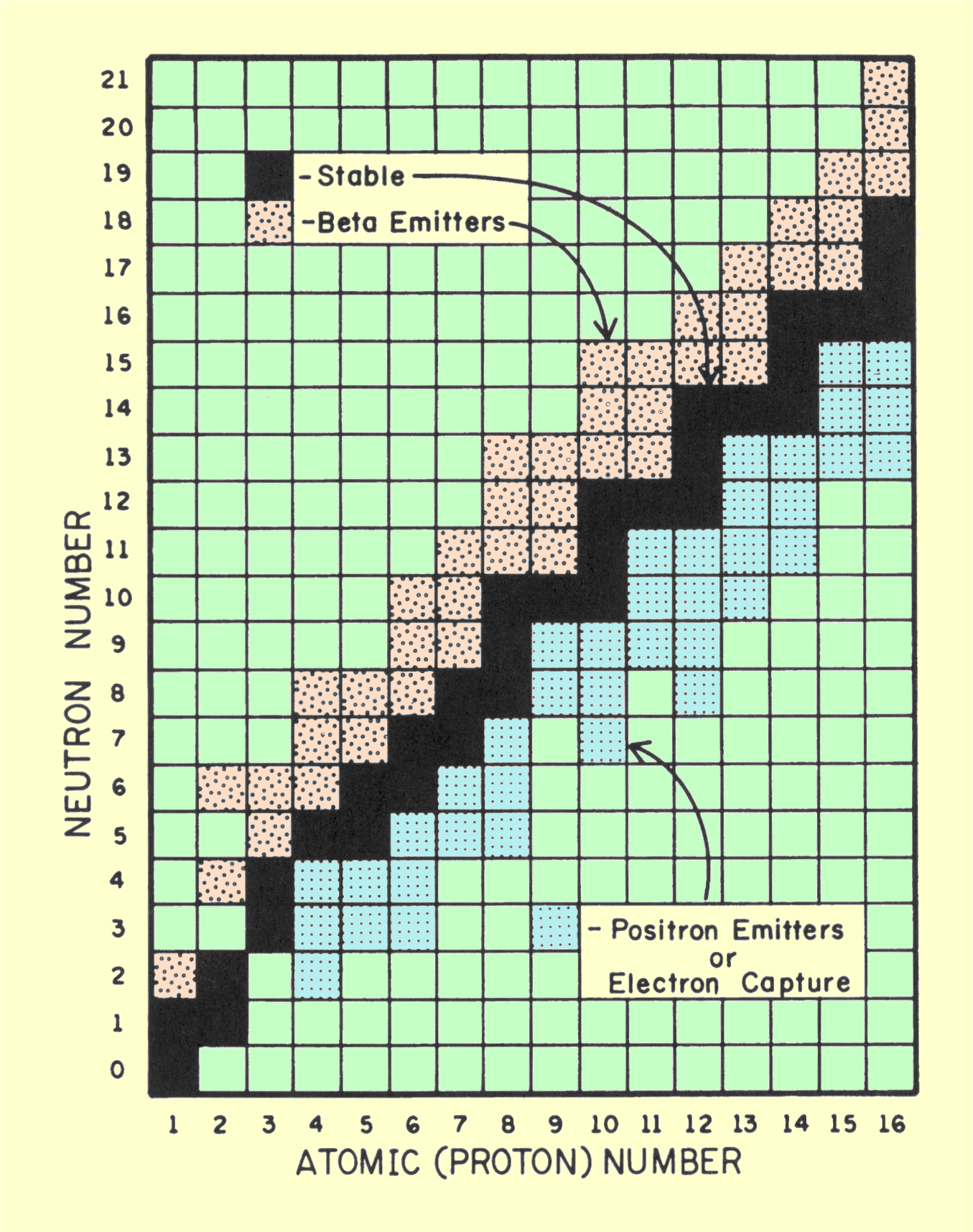
|
|
Nuclide Chart Showing the Relationship between Radioactive and Stable Nuclides
|
|
Beta Emission
|
CONTENTS |
|
A beta transition is illustrated below. We should recall that the
nuclear N/P is too high for stability. During the transition, this
condition is relieved by the conversion of an internal neutron into a
proton, accompanied by the emission, from the nucleus, of an electron. The
electron, or beta particle, has two functions in this transition.
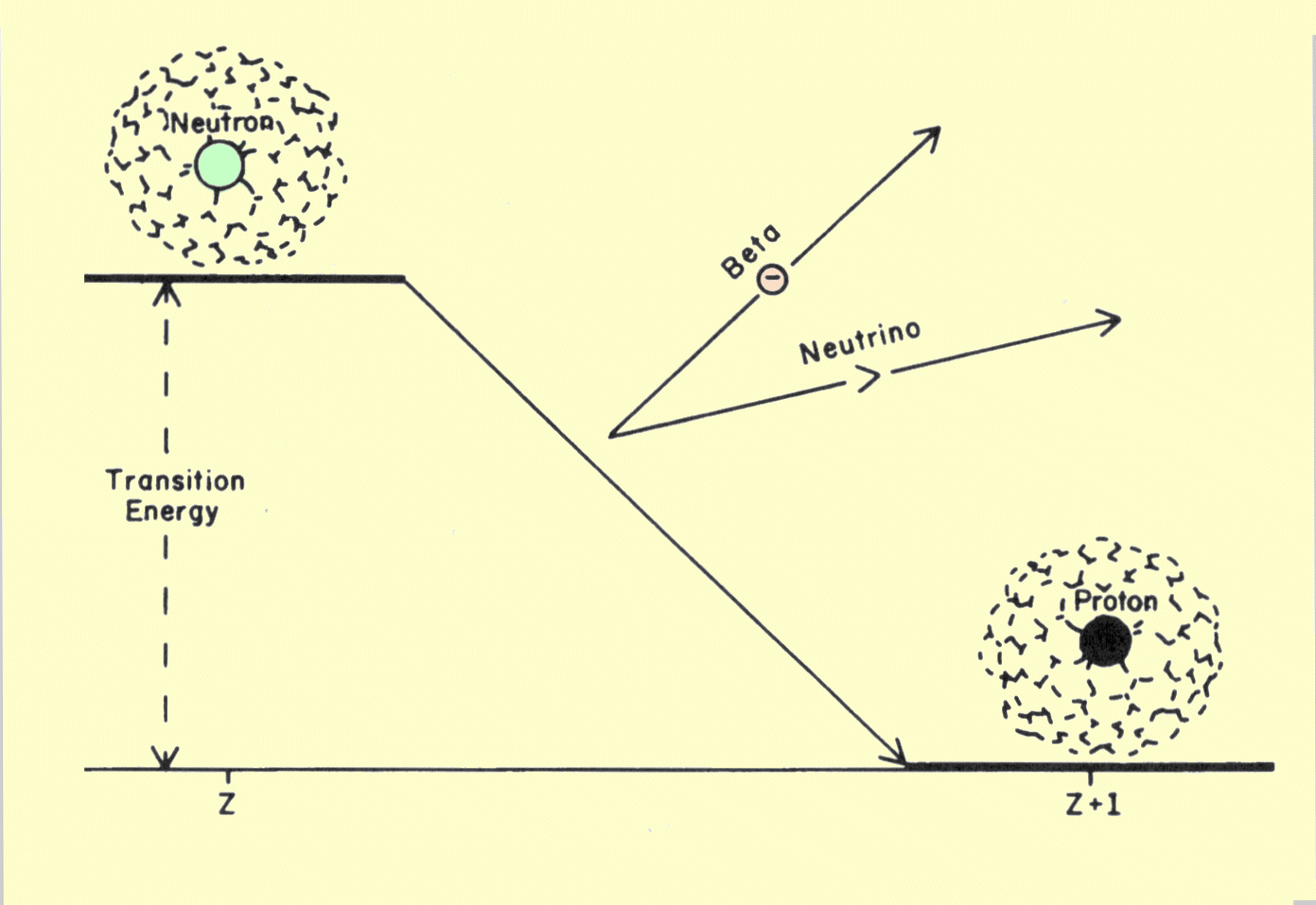
Diagram of a Transition That Produces Beta Radiation
One function is to carry away from the nucleus a one-unit
negative charge so that a neutron (no charge) can be converted into a
proton with a one-unit positive charge. A fundamental principle of physics
is that electrical charge cannot be created or destroyed. The only way to
change the charge on an object, such as a nucleus, is to transfer
electrons to or from the object. The emission of a beta particle causes
the number of protons, and therefore the atomic number, of the nucleus to
increase by one unit. Since the mass number, or total number of neutrons
and protons, is not changed, the transition is isobaric.
The second function of the electron is to carry off a portion
of the energy given up by the nucleus. The energy is carried as kinetic
energy by the electron. But the energy carried by a beta particle is
usually less than the total transition energy given up by the nucleus. The
remaining portion is removed from the nucleus by the emission of a very
small particle known as a neutrino. In each transition, the sum of the
beta and neutrino energy is equal to the transition energy for the
nuclide. Unlike the beta particle, the neutrino is very penetrating and
carries the energy out of the patient's body.
A typical energy spectrum for a beta-emitting nuclide is
shown below. The maximum energy corresponds to the transition
energy. This is the energy a beta particle would have in the few instances
no neutrino is emitted. The average energy value indicates the radiation
dose or energy deposited in the body by the beta radiation. The shape of
the beta energy spectrum varies from nuclide to nuclide. The relationship
between average energy and transition energy depends on the value of the
transition energy and the atomic number of the nuclide. For most
radionuclides encountered in nuclear medicine, the average beta energy is
usually between 25% and 30% of the maximum energy.
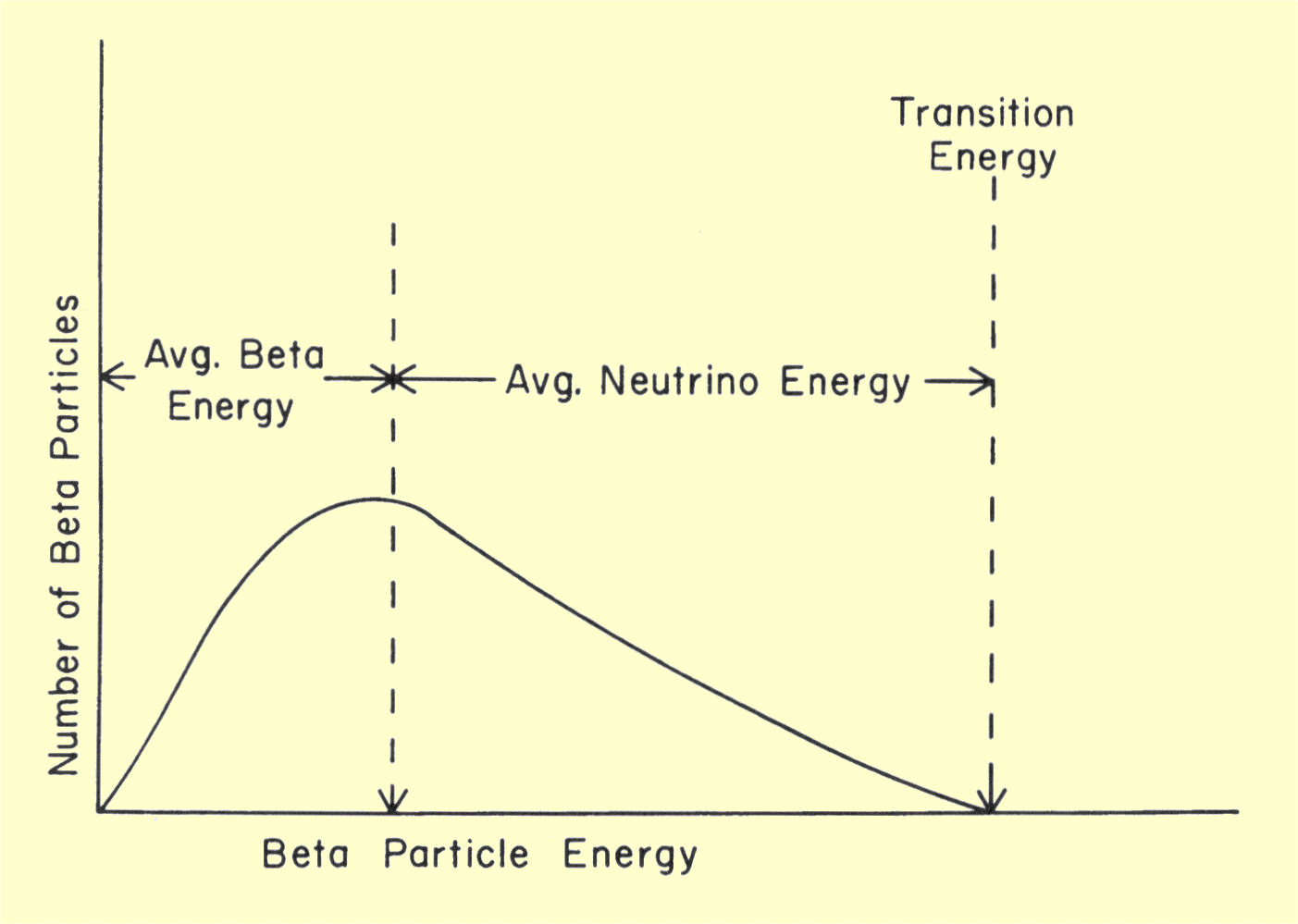
Spectrum of Beta Radiation Energy
|
|
|
|
Positron Emission
|
CONTENTS |
|
Two types of transitions can occur when the nuclear N/P is too low for
stability. One is positron emission. A positron is a small particle that
has essentially the same mass as an electron but has a positive rather
than negative electrical charge. The nuclear transition resulting in the
emission of a positron is illustrated below. The transition energy
is shared between the positron and a neutrino.
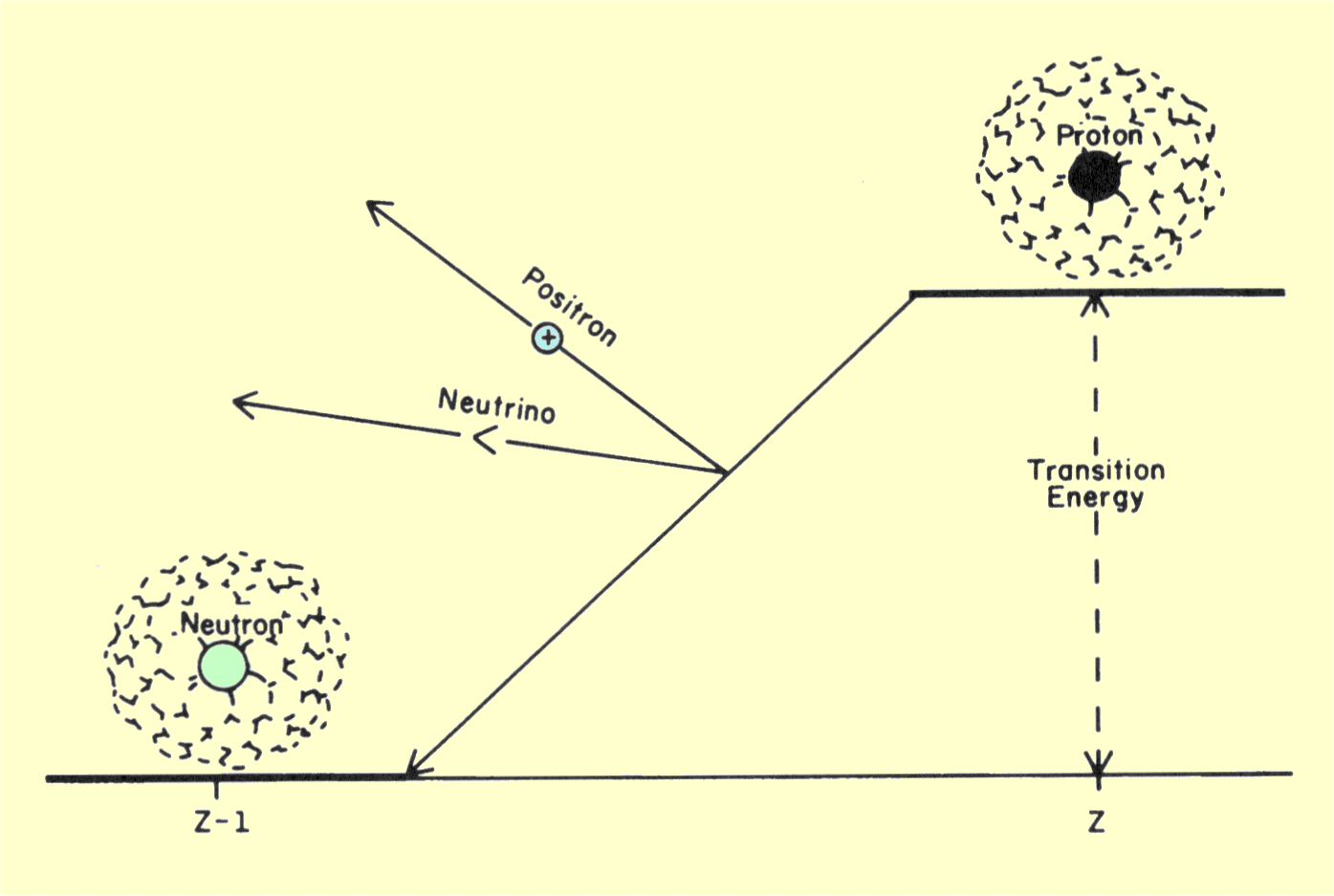
Diagram of a Transition That Produces Positron Radiation
In this transition a proton is converted into a neutron as
the positron particle is formed. Since a neutron is heavier than a proton,
energy is required for this conversion. The energy equivalent to the mass
difference between a neutron and a proton plus the energy equivalent of
the positron mass is approximately 1.8 MeV. This means that the total
transition energy must be at least 1.8 MeV for positron emission to occur.
The positron is the antiparticle of an electron and will
enter an annihilation reaction when the two particles meet. Since
electrons are normally abundant in material, positrons are annihilated
soon after their emission. Positron emitters are useful in nuclear
medicine because of the radiation produced when the positron is
annihilated. The total masses of the positron and the electron are
converted into energy according to the relationship
E
= MC2.
The energy produced is 1.022 MeV emitted as a pair of photons, each with
an energy of 511 keV. Therefore, the radiation from a positron-emitting
material is photons with a characteristic energy of 511 keV. The pair of
photons leave the site traveling in opposite directions. This is useful in
imaging, because it allows the annihilation site to be precisely
determined.
|
|
|
|
Electron Capture
|
CONTENTS |
|
A nucleus can also relieve a low neutron-proton ratio by capturing and
absorbing an electron from a shell. Since most electrons are captured from
the K shell, this process is sometimes referred to as K-capture. Capture
from the L and M shells is possible under some conditions, but does not
occur so frequently as from the K shell. The electron capture process is
illustrated below. When the negative electron enters the nucleus,
the positive charge of one proton is canceled and the proton is converted
into a neutron. This results in the reduction of the atomic number by one
unit. Since the mass number does not change, electron capture is an
isobaric transition. Electron capture often competes with positron
emission; if a nuclide is a positron emitter, some nuclei will emit
positrons and some will capture electrons. The ratio between the two
processes is specific for each nuclide.
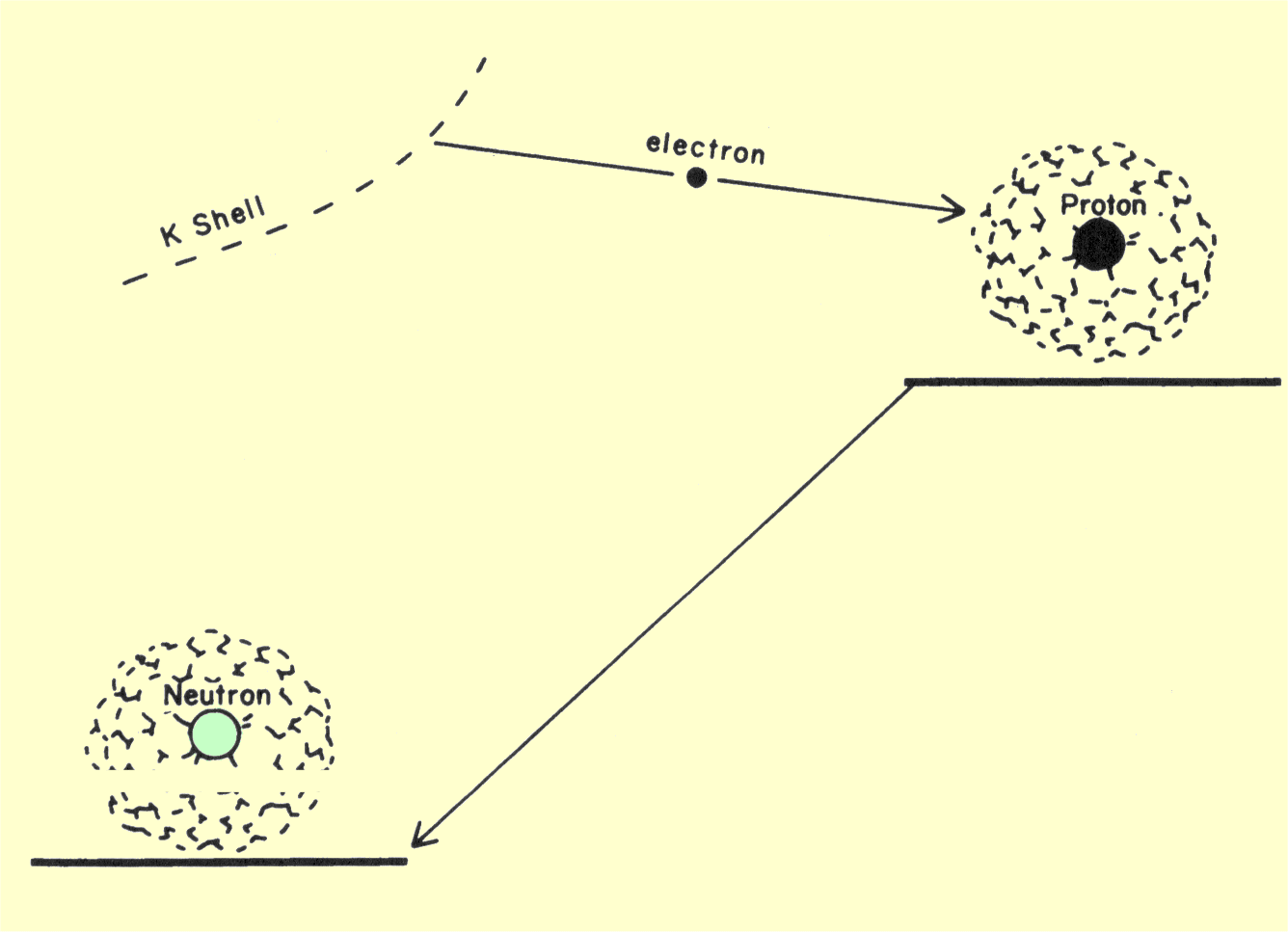
Diagram of a Transition That Produces Electron Capture
In an electron capture transition, radiation is not emitted directly from
the nucleus but results from changes within the electron shells. Electron
capture creates a vacancy in one shell, which is quickly filled by an
electron from a higher energy location. As the electron moves down to the
K shell, it gives off an amount of energy equivalent to the difference in
the binding energy of the two levels. This energy is emitted from the atom
in either characteristic x-ray photons or Auger electrons. Auger electrons
are produced when the energy given up by the electron filling the K-shell
vacancy is transferred to another electron, knocking it out of its shell.
Most Auger electrons have relatively low energies.
Many radionuclides that undergo electron capture are used in nuclear
medicine because the energy of characteristic x-ray photons is ideal for
in-vivo studies.
|
|
|
|
CONTENTS |
|
After a radioactive nucleus undergoes an isobaric transition (beta
emission, positron emission, or electron capture), it usually contains too
much energy to be in its final stable or daughter state. Nuclei in these
intermediate and final states are isomers, since they have the same atomic
and mass numbers. Nuclei in the intermediate state will undergo an
isomeric transition by emitting energy and dropping to the ground state.
|
|
|
|
CONTENTS |
|
In most isomeric transitions, a nucleus will emit its excess energy in the
form of a gamma photon. A gamma photon is a small unit of energy that
travels with the speed of light and has no mass; its most significant
characteristic is its energy. The photon energies useful for diagnostic
procedures are generally in the range of 100 keV to 500 keV.
The energy of a gamma photon is determined by the difference
in energy between the intermediate and final states of the nucleus
undergoing isomeric transition. This difference is the same for all nuclei
of a specific nuclide. However, many nuclides have more than one
intermediate state or energy level. When this is the case, a radionuclide
might emit gamma photons with several different energies. This is
illustrated in below.
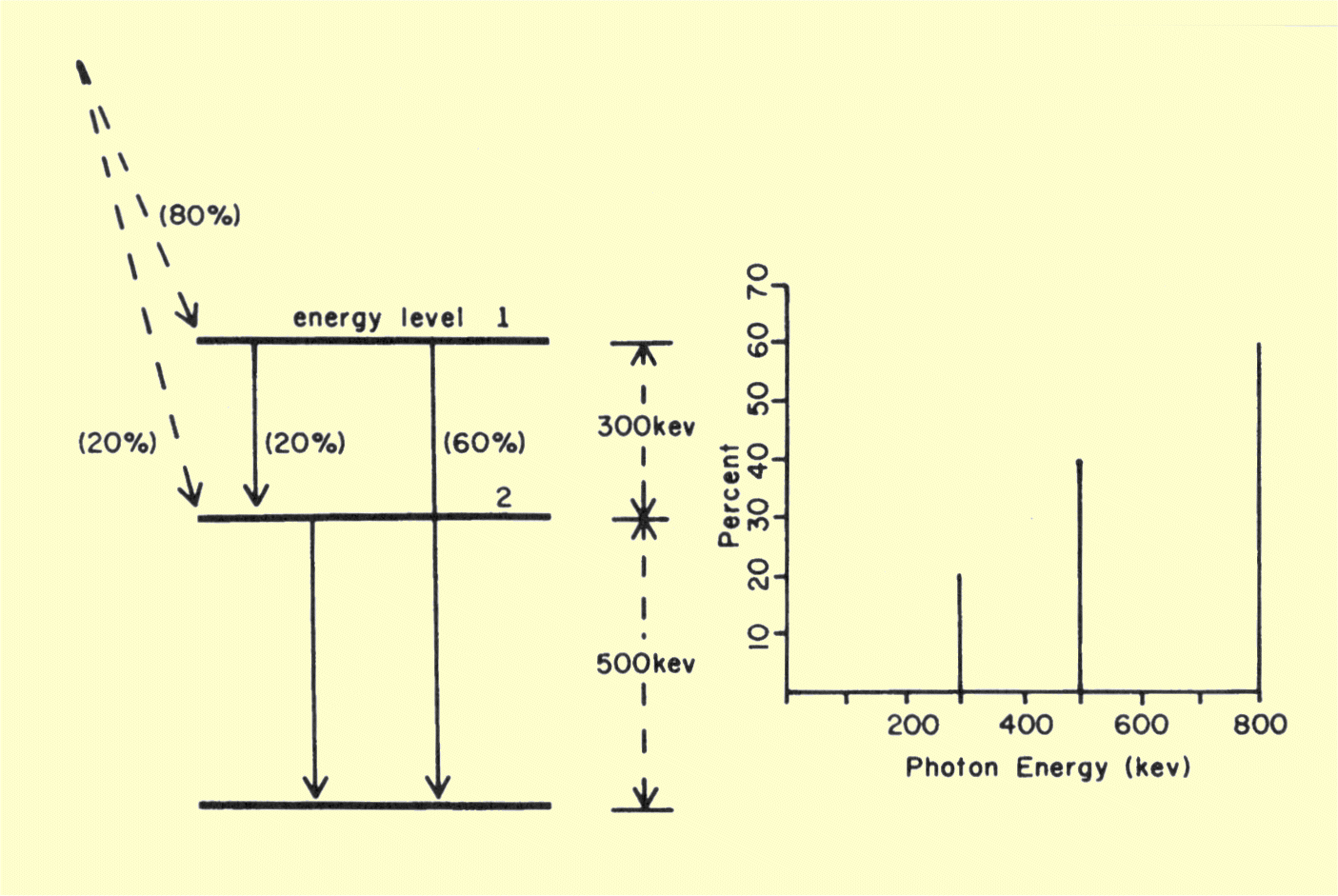
Relationship of Nuclear Energy Levels to the Energy Spectrum of Gamma Photons
The nuclide used in this illustration has two intermediate
states or energy levels. One has an energy 500 keV above the daughter
level, and the other is 300 keV higher than the first. When there are
several different intermediate energy levels, it is common for some nuclei
to go to one level and other nuclei to go to another level during isobaric
transition. This is usually indicated on the transition diagram by showing
the percentage of nuclei that go to
each energy level. In the illustration
considered,
80% of the nuclei go directly to intermediate energy level number 1, and
20% go directly to level number 2. The gamma photons are emitted when the
nuclei move from these intermediate energy levels down to the daughter
nuclide level.
Nuclei that have gone to a specific intermediate energy level might then
go directly to the daughter level or to a lower intermediate level. With
this in mind we can predict the gamma photon energy spectrum produced by
our example nuclide. The spectrum will consist of three discrete energies
as shown above. Sixty percent of the parent nuclei will go to
intermediate energy level number 1 and then directly to the daughter level
800 keV below. Therefore, 60% of the transitions will give rise to an
800-keV photon. Twenty percent of the nuclei that go to energy level 1
will then go to intermediate level number 2 by emitting a 300-keV photon.
Forty percent of the nuclei will go through intermediate energy level
number 2, either directly from the parent or from intermediate level
number l. When these nuclei drop to the daughter energy level, a 500-keV
gamma photon will be emitted. It is the combination of different energy
levels and different transition routes that gives rise to the different
energies in the typical gamma spectrum. For most radionuclides, one or two
gamma energies will account for the vast majority of transitions.
For most nuclides, the time spent by the nucleus in the
intermediate state is extremely short and the isomeric transition appears
to coincide with the isobaric transition. In some nuclides, however, the
nuclei remain in the intermediate state for a longer time. In this case,
the intermediate state is referred to as a metastable state. Metastable
states are of particular interest in nuclear medicine because they make
possible the separation of electron and photon radiation. In a diagnostic
procedure it is
undesirable
to have electron radiation in the body because it contributes to radiation
dosage but not to image formation. By using a nuclide that has already
undergone an isobaric (electron-emitting) transition and is in a
metastable state, it is possible to have a radioactive material that emits
only gamma radiation.
Technetium-99m is a nuclide used in the metastable state. The parent
nuclide is molybdenum, which undergoes an isobaric transition to
technetium-99 in the metastable state. The technetium-99m will later
undergo an isomeric transition to technetium-99.
|
|
|
|
CONTENTS |
|
Under some conditions, the energy from an isomeric transition can be
transferred to an electron within the atom. This energy supplies the
binding energy and expels the electron from the atom. This process is
known as internal conversion (IC) and is an alternative to gamma emission.
In many nuclides, isomeric transitions produce gamma photons and IC
electrons. When an electron is removed from the atom by internal
conversion, a vacancy is created. When the vacancy is filled by an
electron from a higher energy level, energy must be emitted from the atom
as a characteristic x-ray photon or an Auger electron.
The various isobaric and isomeric transitions give rise to a combination
of both photon and particulate radiations. The radiations encountered in
clinical procedures are summarized below. Nuclei with a high N/P
generally produce beta radiation; those with a low N/P produce either
positrons or electron capture. All transitions are usually followed by
either gamma or internal conversion electron emission. Internal conversion
and electron capture lead to x-ray or Auger electron emission.
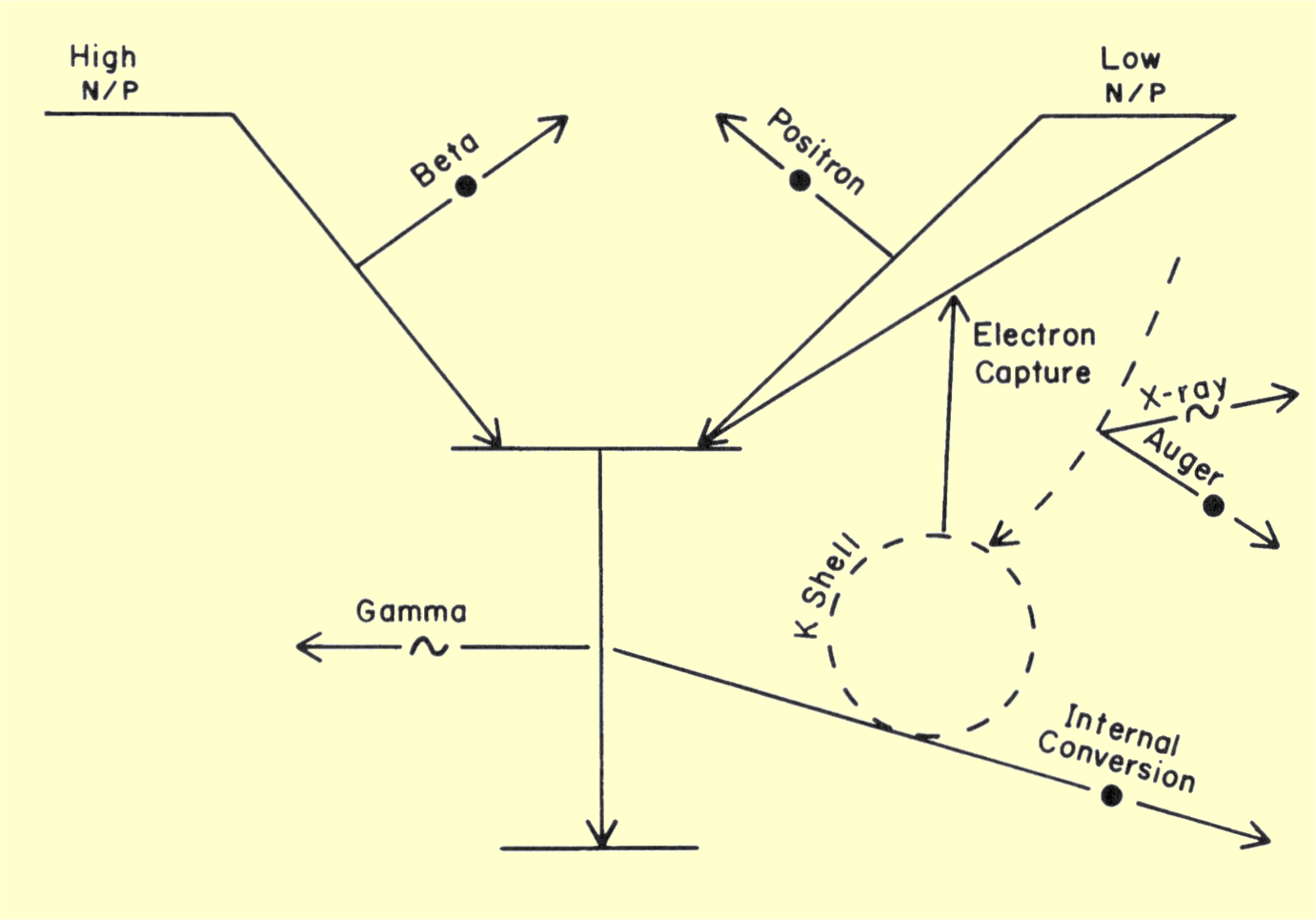
Composite Diagram Showing the Various Nuclear Transitions That Produce Radiation
|
|
|
|
|
CONTENTS |
|
Some radioactive materials emit alpha particles during their
transformation. An alpha particle consists of two neutrons and two
protons. Because of their size and electrical charge, alpha particles are
not good penetrators and deposit their energy very close to their origin.
Because of this, and the fact that alpha particles generally have more
energy than other radiation forms, significant tissue doses can result
from an alpha emitter within the body. Alpha emitters are generally not
used in clinical medicine. An exception is radium, which is often used in
therapeutic procedures. In these procedures the radium is contained in
sealed metal capsules that absorb alpha radiation and let gamma radiation
through to the tissue.
The transitions and principal radiations emitted by many
nuclides used in clinical medicine are shown in the following table.
|
Radionuclides Used in Nuclear Medicine
| Element |
A |
Z |
T1/2 |
Transition
|
Radiation |
Yield |
Energy (keV) |
| Hydrogen |
3 |
1 |
12.3 yr |
Beta |
Beta |
1.0 |
57 |
| Carbon |
11 |
6 |
20.3 min |
Positron |
Positron |
1.0 |
394 |
| Annihilation |
2.0 |
511 |
| Nitrogen |
13 |
7 |
10 min |
Positron |
Positron |
1.0 |
488 |
| Annihilation |
2.0 |
511 |
| Carbon |
14 |
6 |
5730 yr |
Beta |
Beta |
1.0 |
49 |
| Oxygen |
15 |
8 |
2 min |
Positron |
Positron |
1.0 |
721 |
| Annihilation |
2.0 |
511 |
| Fluorine |
18 |
9 |
109 min |
Positron |
Positron |
0.97 |
250 |
| EC (3%) |
Annihilation |
1.94 |
511 |
| Chromium |
51 |
24 |
27.7 d |
EC |
Gamma |
0.10 |
320 |
| Cobalt |
57 |
27 |
270 d |
EC |
Gamma |
0.86 |
122 |
| Gamma |
0.10 |
136 |
| Iron |
59 |
26 |
45 d |
Beta |
Beta |
0.52 |
150 |
| Beta |
0.46 |
81 |
| Gamma |
0.55 |
1099 |
| Gamma |
0.44 |
1292 |
| Gallium |
67 |
31 |
78.1 hr |
EC |
Gamma |
0.38 |
93 |
| Gamma |
0.24 |
185 |
| Gamma |
0.16 |
300 |
| IC Electron |
0.28 |
84 |
| Zinc |
69m |
30 |
13.8 hr |
Isomeric |
Gamma |
0.95 |
440 |
| Selenium |
75 |
34 |
120 d |
EC |
Gamma |
0.16 |
121 |
| Gamma |
0.54 |
136 |
| Gamma |
0.57 |
265 |
| Gamma |
0.24 |
280 |
| Gamma |
0.12 |
400 |
| Strontium |
85 |
38 |
65.1 d |
EC |
Gamma |
0.99 |
514 |
| Strontium |
87m |
38 |
2.8 hr |
EC and Isomeric |
Gamma |
0.83 |
388 |
| IC electron |
0.14 |
372 |
| Technetium |
99m |
43 |
6.0 hr |
Isomeric |
IC electron |
0.09 |
119 |
| Gamma |
0.88 |
141 |
| Indium |
111 |
49 |
2.8 d |
EC |
Gamma |
0.90 |
172 |
| Gamma |
0.94 |
247 |
| Iodine |
123 |
53 |
13 hr |
EC |
IC electron |
0.13 |
127 |
| Gamma |
0.84 |
159 |
| X-Ray |
0.71 |
27 |
| Iodine |
125 |
53 |
60.2 d |
EC |
X-Ray |
1.15 |
27 |
| Iodine |
131 |
53 |
8.0 d |
Beta |
Beta |
0.90 |
192 |
| Gamma |
0.82 |
364 |
| Gamma |
0.07 |
637 |
| Xenon |
133 |
54 |
5.31 d |
Beta |
Beta |
0.98 |
101 |
| IC electron |
0.53 |
45 |
| Gamma |
0.36 |
81 |
| Ytterbium |
169 |
70 |
32 d |
EC |
IC electron |
0.47 |
108 |
| IC electron |
0.15 |
196 |
| Gamma |
0.45 |
63 |
| Gamma |
0.11 |
130 |
| Gamma |
0.17 |
177 |
| Gamma |
0.26 |
198 |
| X-Ray |
0.78 |
51 |
| Mercury |
197 |
80 |
65 hr |
EC |
IC electron |
0.56 |
64 |
| IC electron |
0.19 |
75 |
| Gamma |
0.25 |
77 |
| X-Ray |
0.36 |
69 |
| Gold |
198 |
79 |
2.69 d |
Beta |
Beta |
0.99 |
316 |
| Gamma |
0.96 |
412 |
|
|
|
CONTENTS |
|
Some
radionuclides occur in nature but are generally not suitable for clinical
studies. Most are made by bombarding a nucleus with a particle such as a
neutron or a proton. Beta emitters are created by neutron bombardment, and
positron emitters and nuclides that undergo electron capture are created
by bombardment with positive particles such as protons. Neutrons can be
obtained from nuclear reactors or accelerators. Positive particles are
obtained from accelerators, usually cyclotrons.
|
|
|










