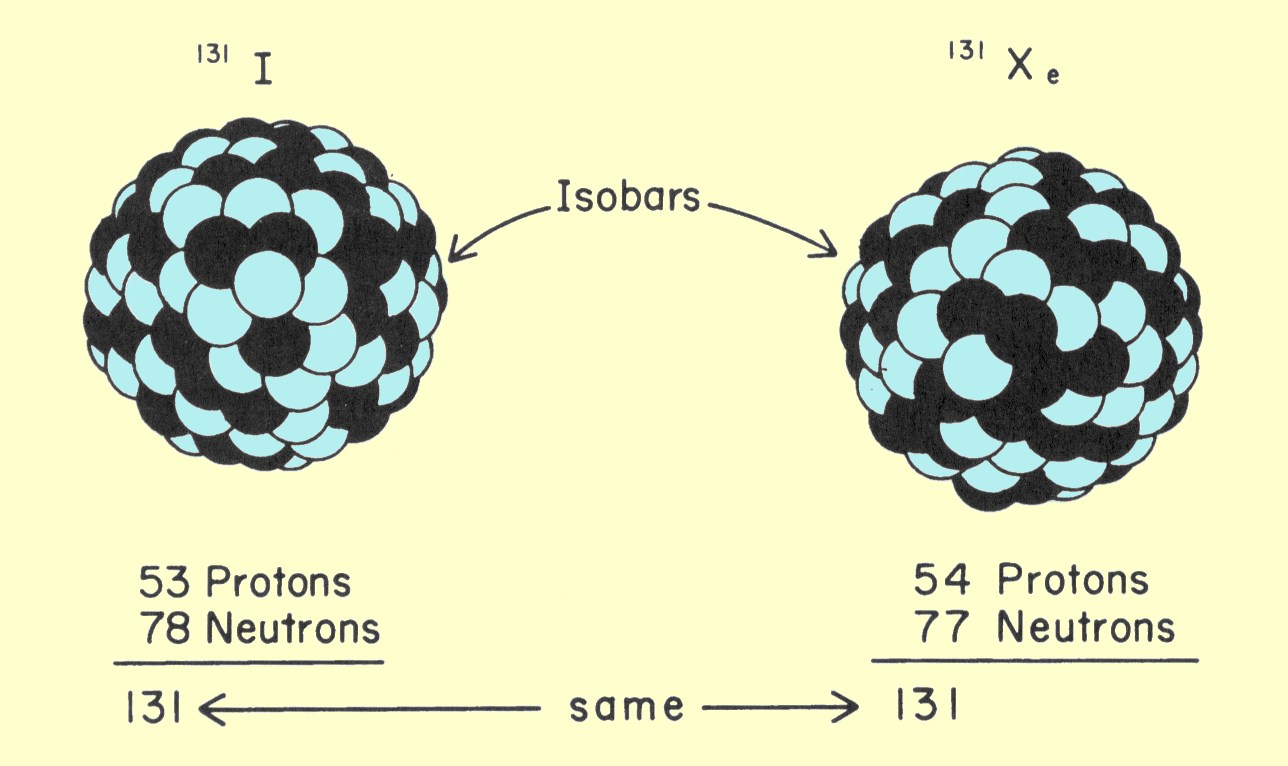
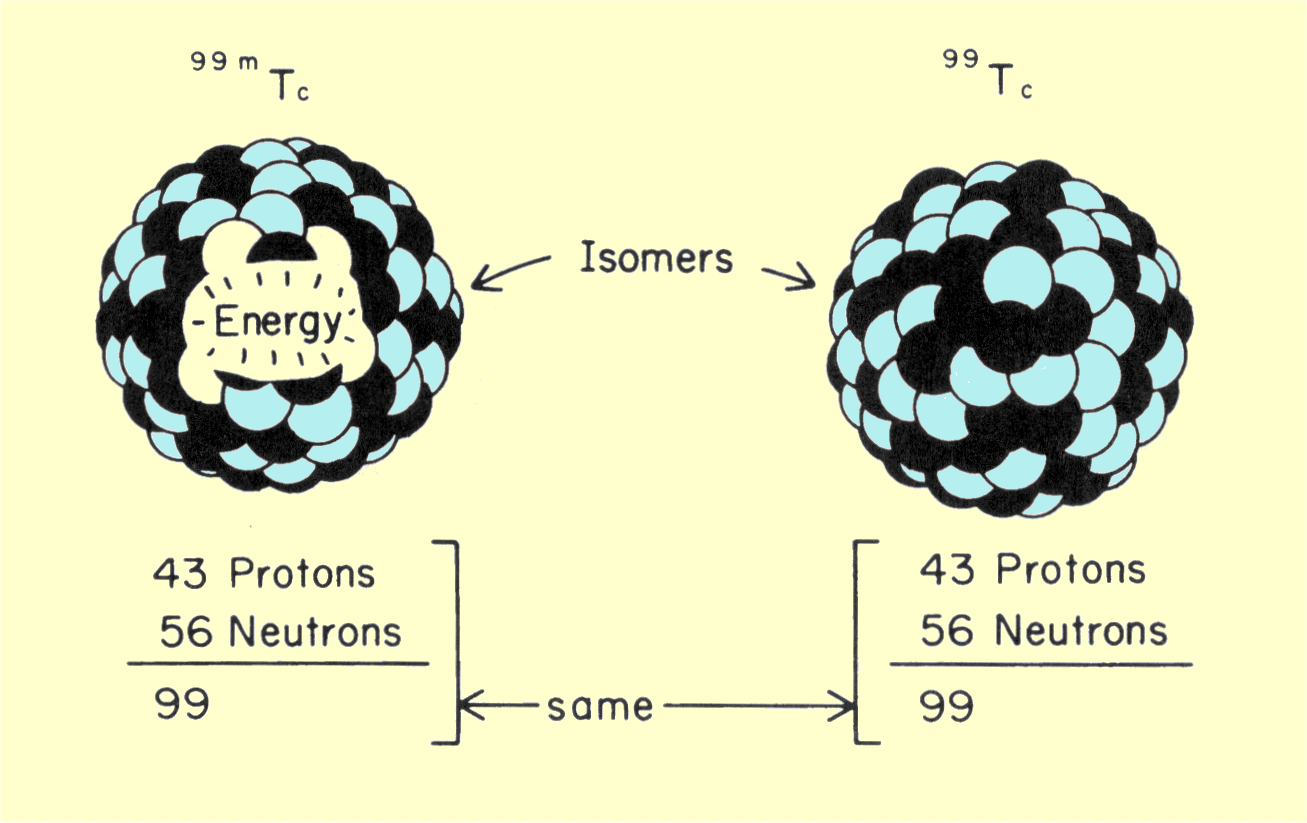
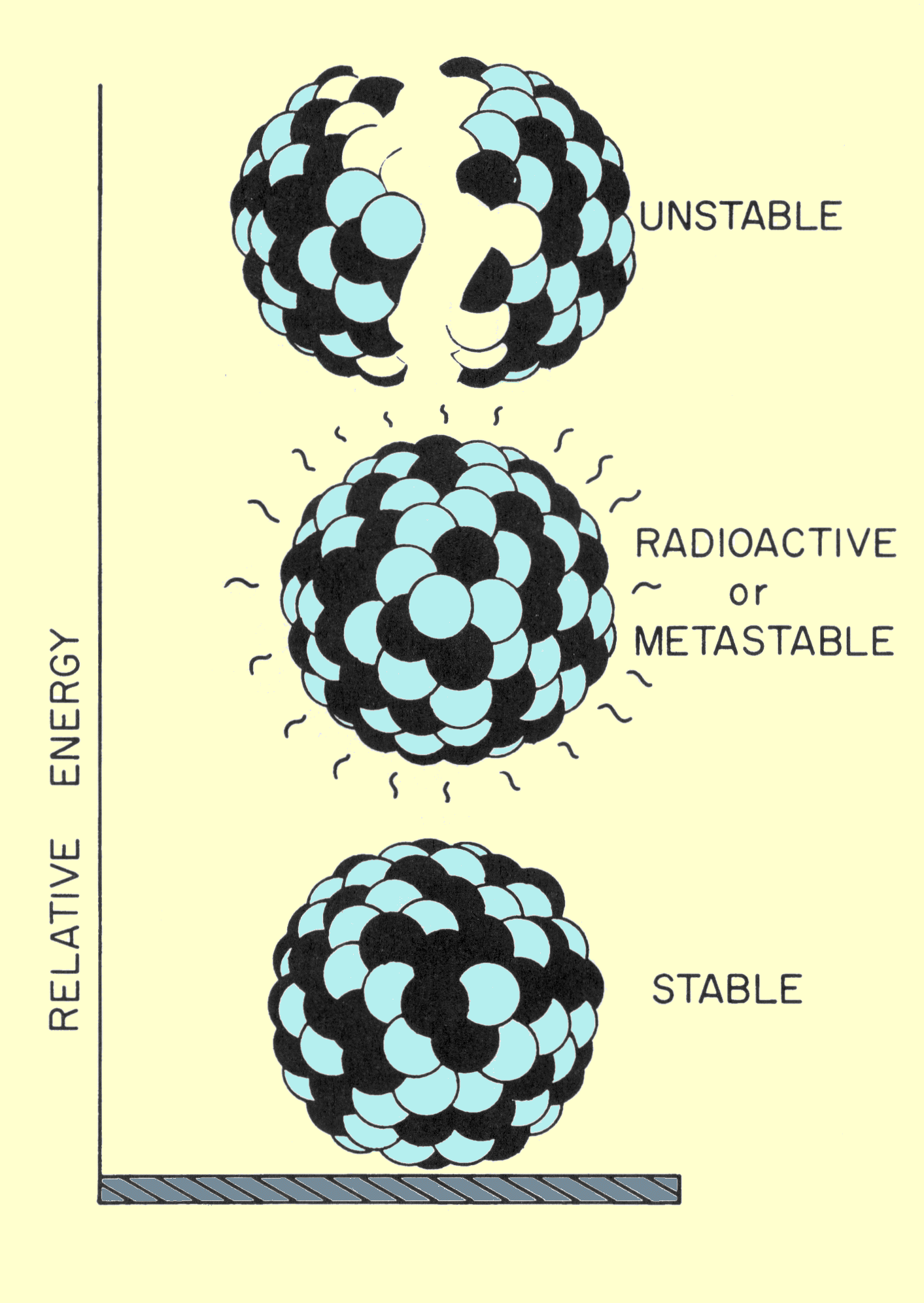
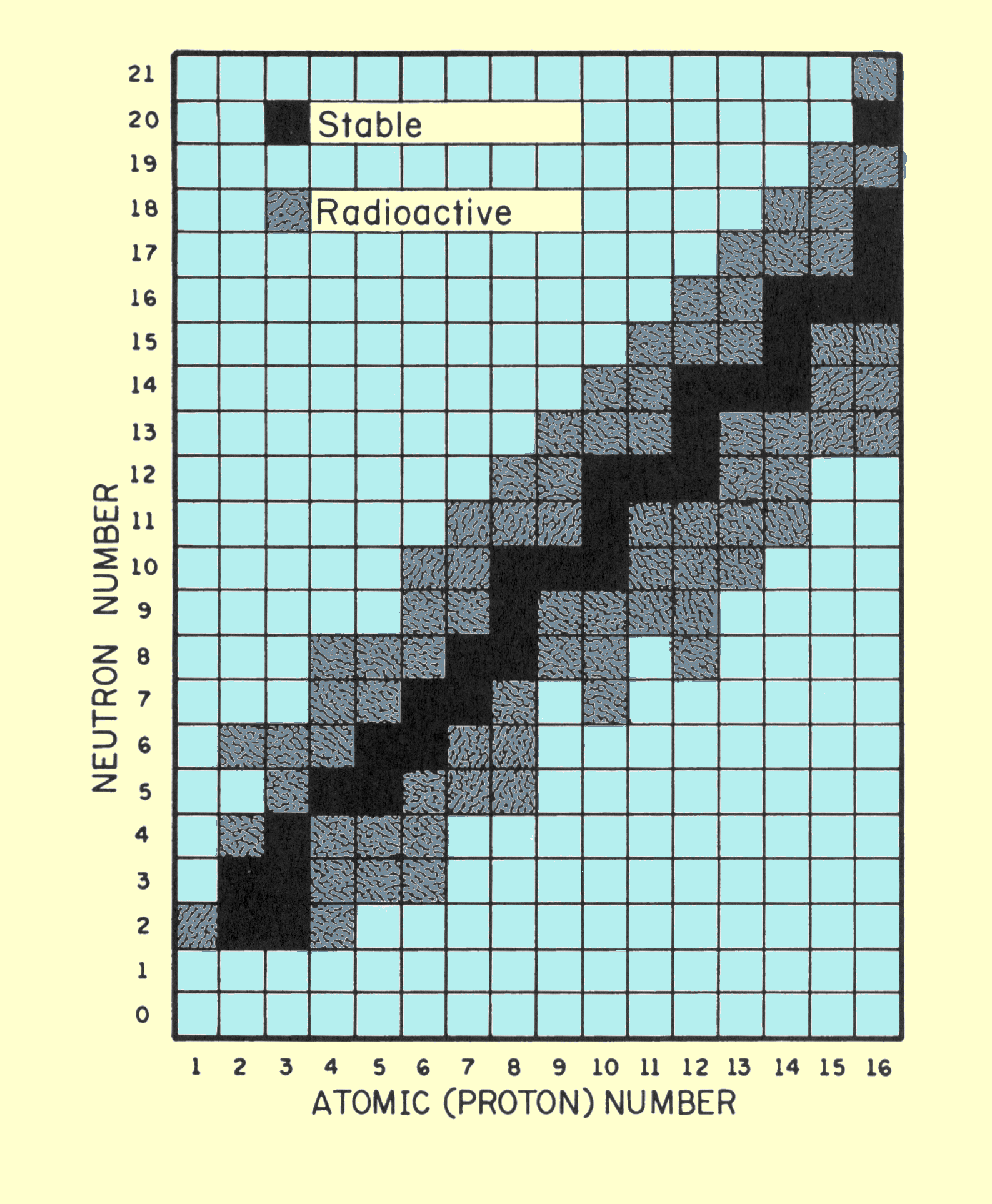
|
Photons are absorbed when they collide with electrons. As a photon passes through matter, its chances of being absorbed generally depend on the concentration of available electrons within the material. The concentration, or number of electrons per cubic centimeter, is given by
Electrons per cc = rN(Z/A).
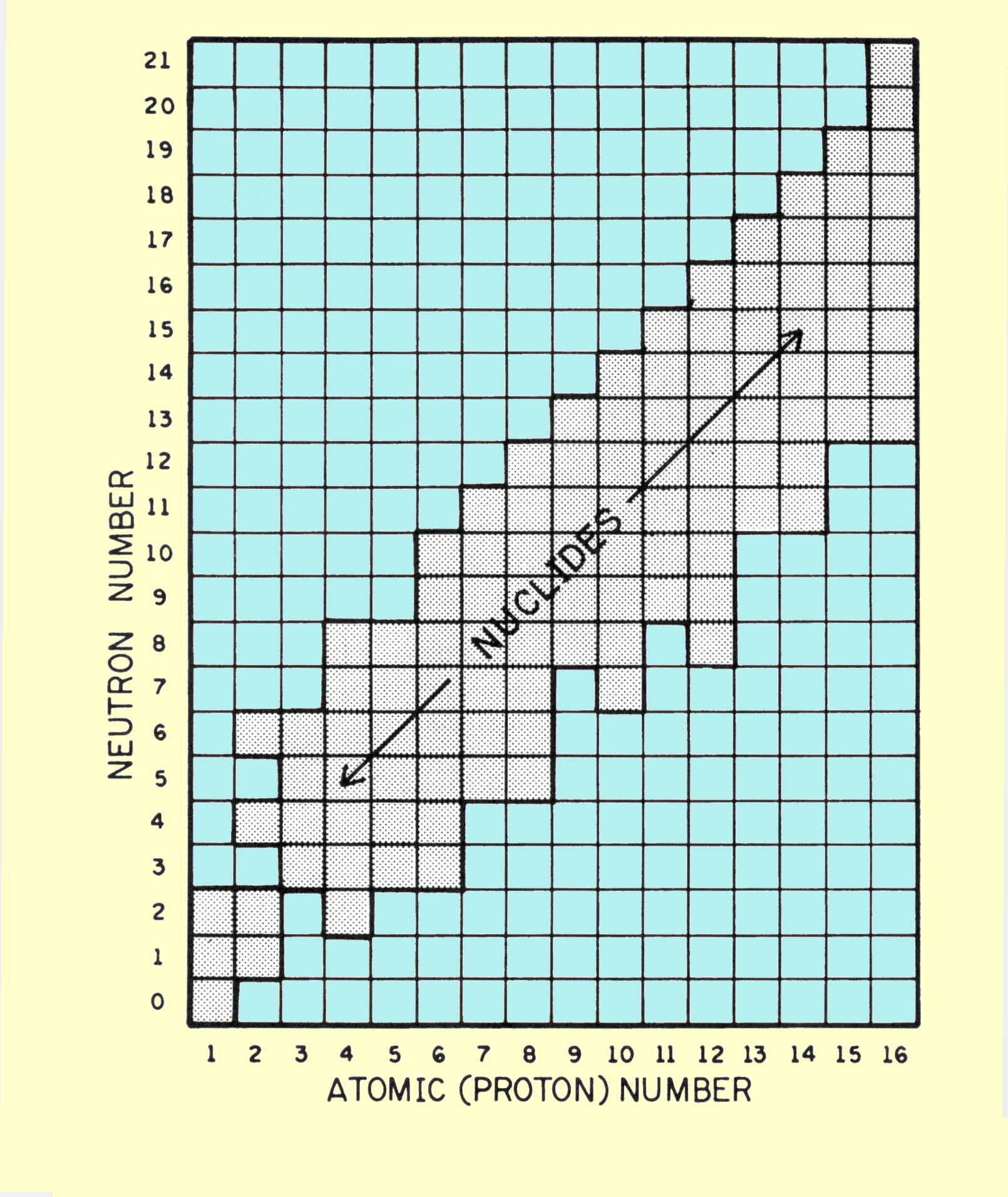
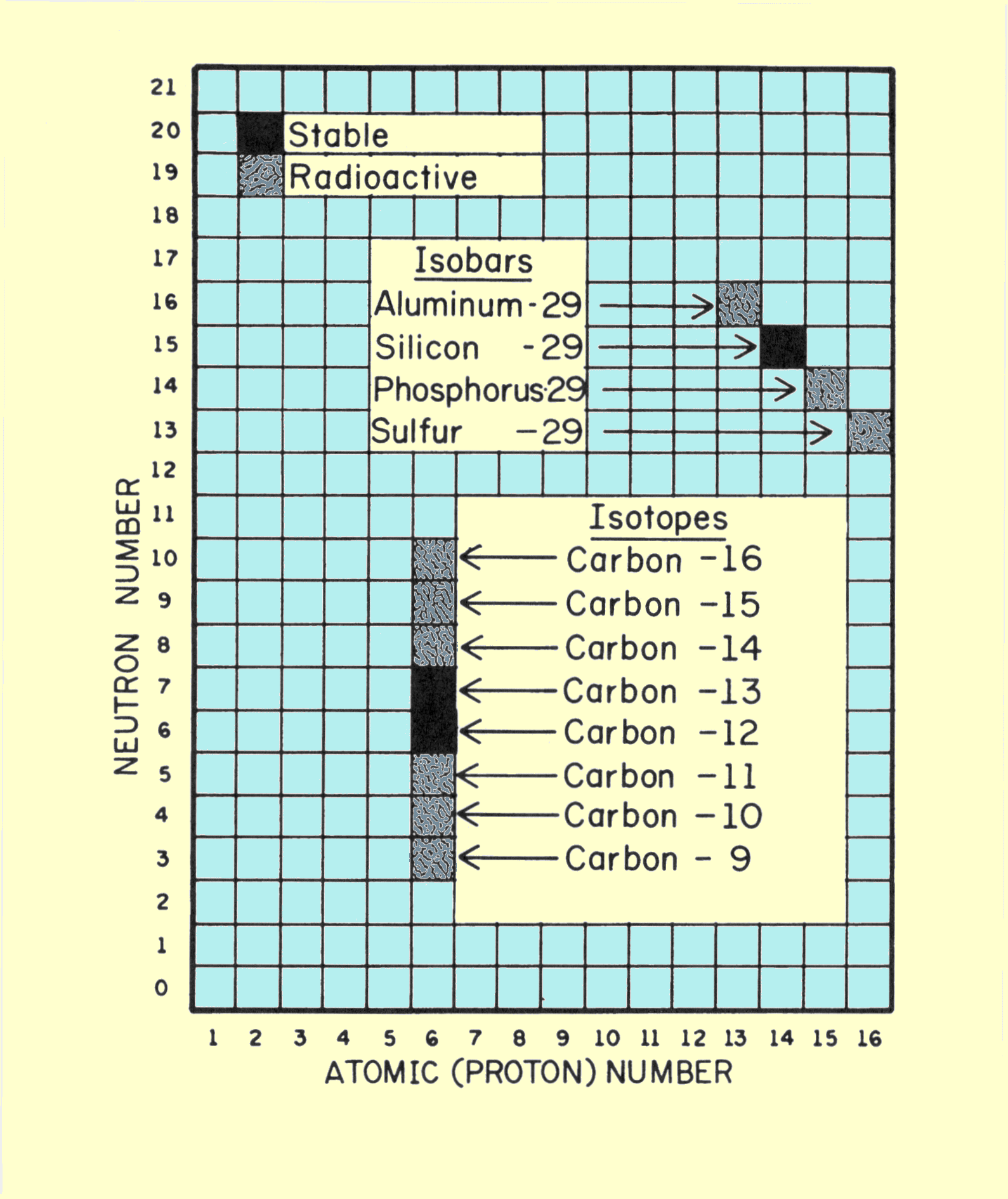
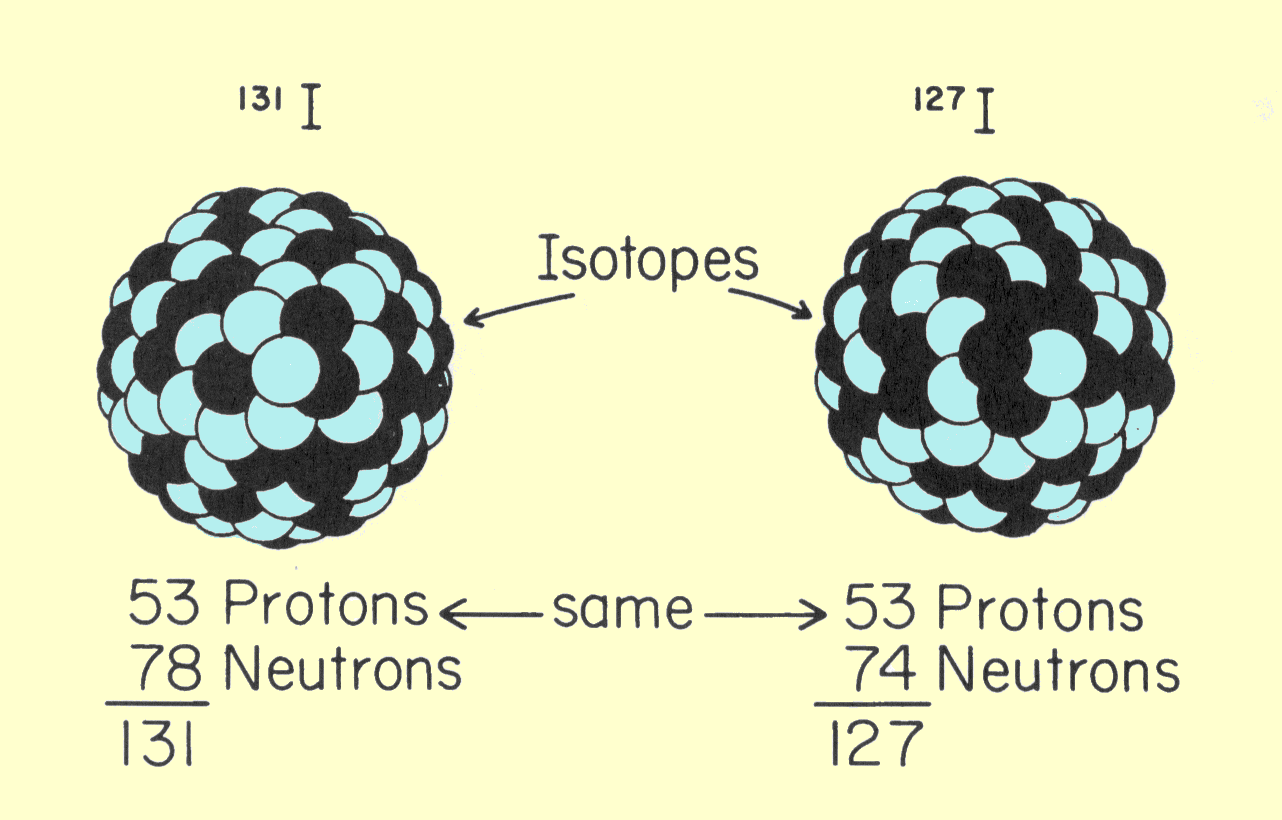
This relationship is the number of atoms per cubic centimeter multiplied by the atomic number, which is the number of electrons per atom. Several comments concerning this relationship are in order. Avogadro's number, N, always has the same value and obviously does not change from element to element. Z and A have unique values for each chemical element. It should be noticed, however, that the number of electrons per cubic centimeter depends only on the ratio of Z to A. The elements with lower atomic numbers have approximately one neutron for each proton in the nucleus. The value of Z/A is approximately 0.5. As the atomic number and atomic weight increase, the ratio of neutrons within the nucleus also increases. This produces a decrease in the Z/A ratio, but this change is relatively small. Lead, which has an atomic number of 82 and an atomic weight of 207, has a Z/A ratio of 0.4. For most material encountered in x-ray applications, the Z/A ratio varies by less than 20%. The single exception to this is hydrogen. Normal hydrogen contains no neutrons and has a nucleus that consists of a single proton. The Z/A ratio, therefore, has a value of l.
Since Avogadro's number is constant, and the Z/A ratio is essentially constant, the only factor that can significantly alter the electron concentration is the density of the material. Most materials, especially pure elements, have more or less unique density values. In compounds and mixtures, the density depends on the relative concentration of the various elements.
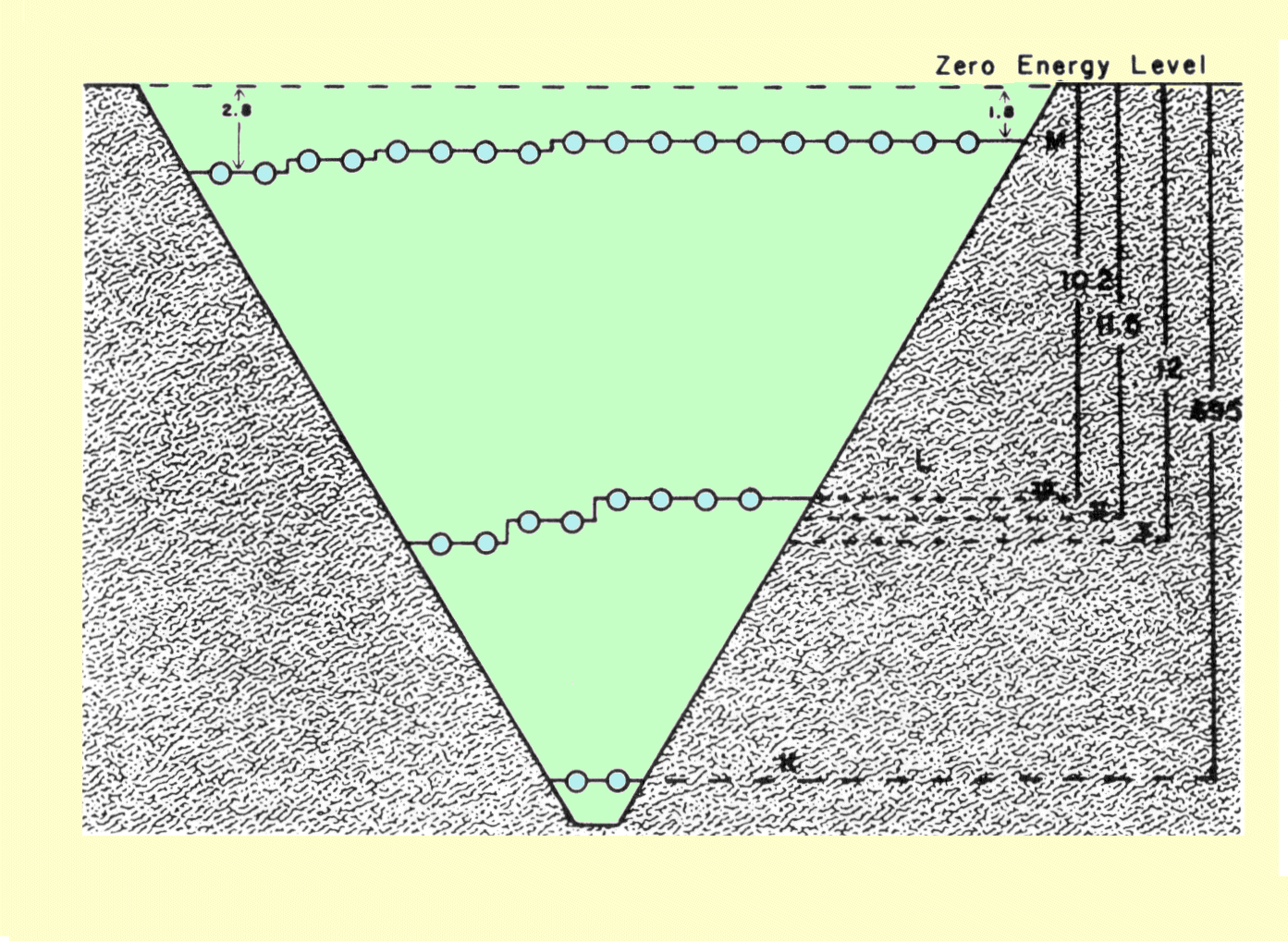
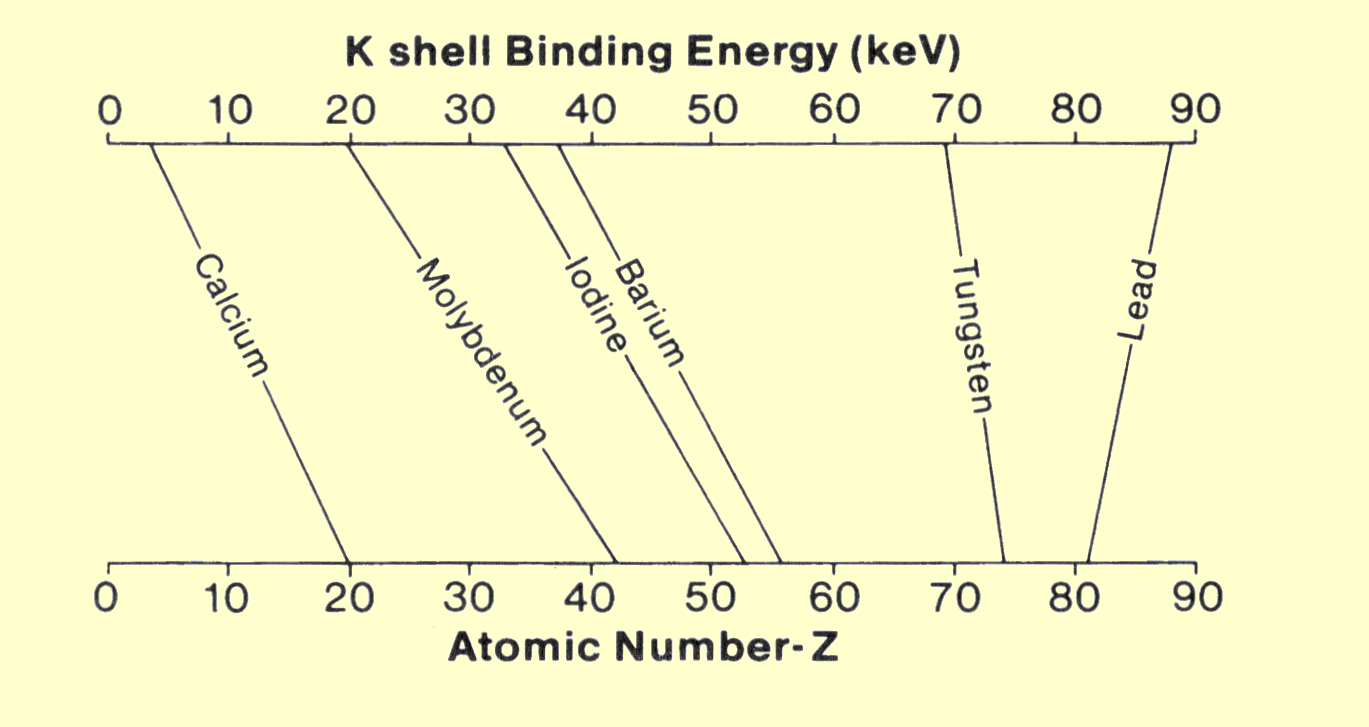
The fact that electron concentration does not significantly change with atomic number might suggest that atomic number has little to do with electron-x-ray interactions. This is, however, not the case. As x-ray photons pass through matter, the chance of interaction depends not only on electron concentration, but also on how firmly the electrons are bound within the atomic structure. Certain types of interactions occur only with firmly bound electrons. Since the binding energy of electrons increases with atomic number, the concentration of highly bound electrons increases significantly with increased atomic number.
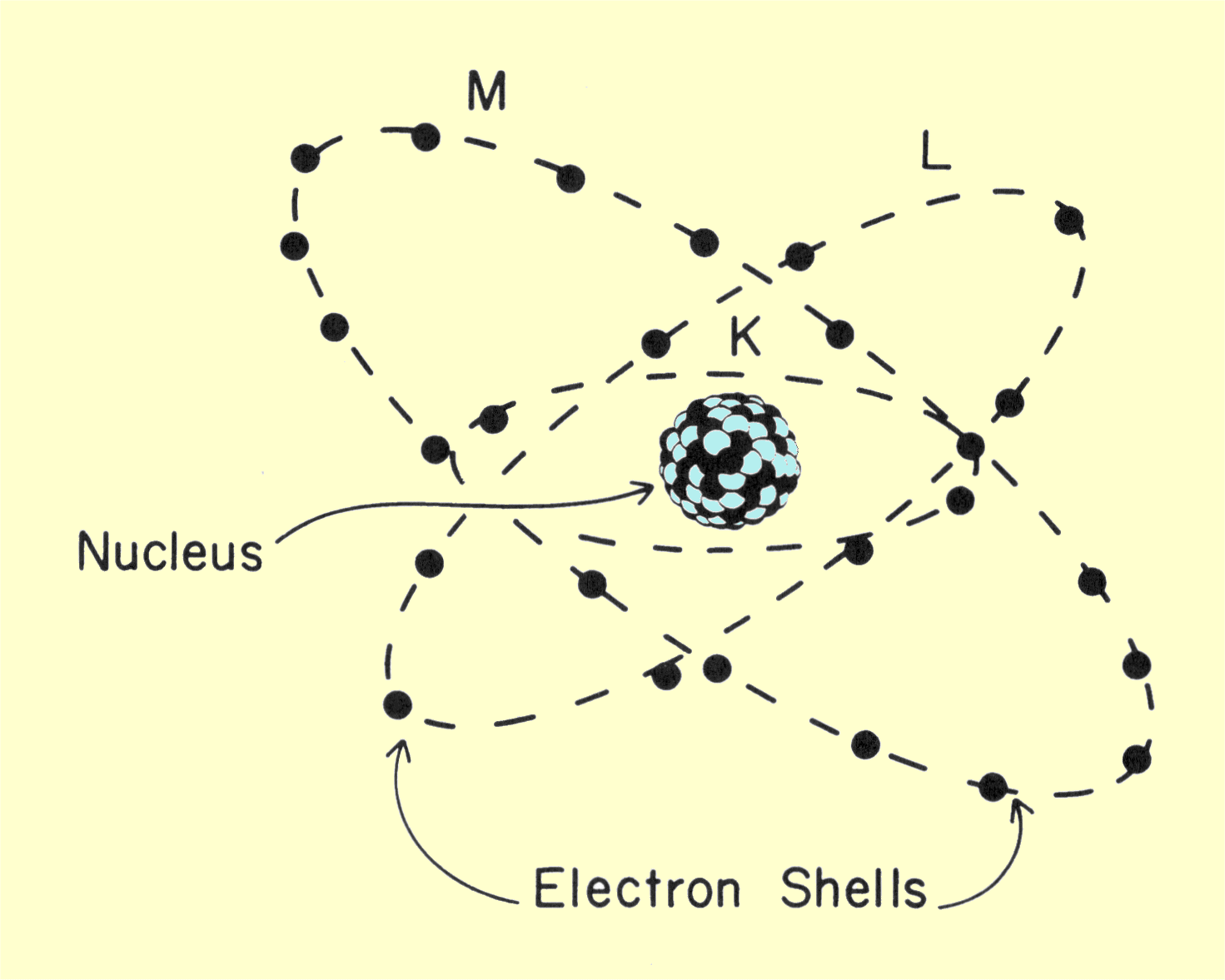
Atomic number is essentially a characteristic of the atom and has a value that is unique to each chemical element. Many materials, such as human tissue, are not a single chemical element, but a conglomerate of compounds and mixtures. With respect to x-ray interactions, it is possible to define an effective atomic number,
Zeff, for compounds and mixtures. The effective atomic number is given by
___________________________
Zeff = 2.94√f1Z12.94 +
f2Z22.94 +
f3Z32.94
+ - - .
In this relationship, f is the fraction of the total number of electrons associated with each element. The exponent, 2.94, is derived from the relationship between x-ray interactions and atomic number, which is discussed later.
Water, which is a major component of the human body, can be used to demonstrate the concept of effective atomic number. The water molecule contains two hydrogen atoms, which have one electron each, and one oxygen atom with eight electrons. The electron fractions, f, are therefore 0.2 for hydrogen and 0.8 for oxygen. Substitution of these values in the above relationship gives an effective atomic number for water of
______________________
Z = 2.94√(0.2
x 1)2.94 +
(0.8 x 8)2.94
= 7.42.
In many x-ray systems, a variety of materials are involved in x-ray interactions. Many of these materials are listed in
the following table, along with their principal physical characteristics and relationship to the x-ray system.
|