
Chapter 3
Nuclear Magnetic Resonance
 |
Chapter 3 |
| Link to Book Table of Contents | Chapter Contents Shown Below |
|
Mind Map Summary |
||
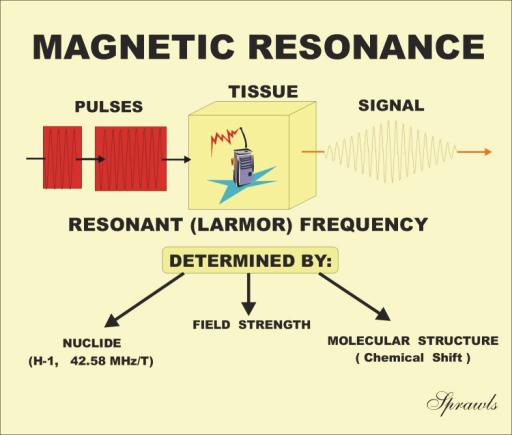
When certain materials, such as tissue, are placed in
a strong magnetic field, two things happen. The materials take on a
resonant characteristic
and they become
magnetized. In this chapter we will consider
the resonant characteristic. In Chapter 4 we will study the magnetization
effect. Resonance
means the materials can absorb and then re-radiate RF
radiation at a specific frequency, like a radio receiver-transmitter, as
illustrated in Figure 3-1. It is actually the nuclei of the atoms that resonate.
The phenomenon is generally known as nuclear magnetic resonance (NMR). The
resonant frequency of material such as tissue is typically in the RF
range so that the emitted radiation is in the form of radio signals. The
specific resonant frequency is determined by three factors as shown in the
illustration and will be described in detail later. The characteristics of the
RF signals emitted by the material are determined by certain physical and
chemical characteristics of the material. The RF signals produced by the NMR
process can be displayed either in the form of images (MRI) or as a graph
depicting chemical composition (MR spectroscopy).
|
Figure 3-1. The concept of Nuclear
Magnetic Resonance (NMR). |
 |
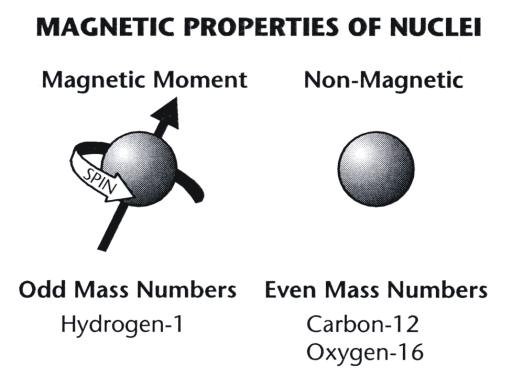
Materials that participate in the MR process must contain nuclei with specific
magnetic properties. In order to interact with a magnetic field, the nuclei
themselves must be small magnets and have a magnetic property or magnetic
moment, as shown in Figure 3-2. The magnetic characteristic of an individual
nucleus is determined by its neutron-proton composition. Only certain nuclides
with an odd number of neutrons and protons are magnetic. Even though most
chemical elements have one or more isotopes with magnetic nuclei, the number of
magnetic isotopes that might be useful for either imaging or in vivo
spectroscopic analysis is somewhat limited. Among the nuclides that are magnetic
and can participate in an NMR process, the amount of signal produced by each
nuclide varies considerably.
|
Figure 3-2. Magnetic and non-magnetic
nuclei. |
 |
Protons
and neutrons that make up a nucleus have an intrinsic angular momentum or
spin. Pairs of protons and neutrons align in such a way that their spins
cancel. However, when there is an odd number of protons or neutrons (odd mass
numbers), some of the spins will not be canceled and the total nucleus will have
a net spin characteristic. It is this spinning characteristic of a particle with
an electric charge (the nucleus) that produces a magnetic property known as the
magnetic moment.
It is for this reason that magnetic nuclei, such as protons, are often
referred to as spins.
The magnetic property, or magnetic moment, of a nucleus has a specific
direction. In Figure 3-2, the direction of the magnetic moment is
indicated by an arrow drawn through the nucleus.
During the imaging process, the body section is divided into an array of
individual volume elements, or voxels. It is the signal intensity from each
voxel that determines image quality. The signal is produced by the magnetic
nuclei within each voxel. Therefore, signal intensity is, in general,
proportional to the quantity of magnetic nuclei within an individual voxel. We
now consider the factors that affect the number of magnetic nuclei within an
individual voxel
In comparison to all other nuclides, hydrogen produces an extremely
strong signal. This results from its high values for each of the three
contributing factors.
Of the three factors, only the concentration, or density, of the nuclei
varies from point to point within an imaged section of tissue. The quantity is
often referred to as proton density and is the most fundamental tissue
characteristic that determines the intensity of the RF signal from an individual
voxel, and the resulting pixel brightness. In most imaging situations, pixel
brightness is proportional to the density (concentration) of nuclei (protons) in
the corresponding voxel, although additional factors, such as relaxation times,
modify this relationship.
Protons in solids, such as the tabletop and bone, do not produce signals.
Signals come only from protons in molecules that are free to move, as in a
liquid state.
The most abundant isotopes of the four elements are hydrogen-1,
carbon-12, nitrogen-14, and oxygen-16. Note that the mass number of hydrogen (1)
is odd while the mass numbers of the other three (12, 14, 16) are even.
Therefore, hydrogen is the only one of these four isotopes that has a strong
magnetic nucleus. The nucleus of the hydrogen-1 atom is a single proton. Among
all the chemical elements, hydrogen Spins
is
unique in that it occurs in relatively high concentrations in most tissues, and
the most abundant isotope (H-1) has a magnetic nucleus.
Other elements, such as sodium, phosphorus, potassium, and magnesium, are
present in very low concentrations. Calcium is concentrated in bone or localized
deposits.
Within this group of elements with low tissue concentrations are several
with magnetic nuclei. These include fluorine-19, sodium-23, phosphorus-31, and
potassium-39.
Table 3-1. Relative Sensitivities of Some Magnetic Nuclides
|
Nuclide |
Sensitivity |
|
Hydrogen-1
Fluorine-19
Sodium-23
Phosphorous-1 |
1.0
0.83
0.093
0.066
|
In
summary, hydrogen has a lot going for it: 1) a high tissue concentration; 2) the
most abundant isotope (H-1) is magnetic; and 3) it produces a relatively strong
signal compared to an equal concentration of other nuclei. That is why hydrogen
is the only element that is imaged with conventional MRI systems.
The NMR
process is a series of interactions involving the magnetic nuclei, a magnetic
field, and RF energy pulses and signals.
In the absence of a strong magnetic field, magnetic moments of nuclei are
randomly oriented in space. Many nuclei in tissue are not in a rigid structure
and are free to change direction. In fact, nuclei are constantly tumbling, or
changing direction, because of thermal activity within the material; in this
case, tissue.
When a material containing magnetic nuclei is placed in a magnetic field,
the nuclei experience a torque that encourages them to align with the direction
of the field. In the human body, however, thermal energy agitates the nuclei and
keeps most of them from aligning parallel to the magnetic field. The number of
nuclei that do align with the magnetic field is proportional to the field
strength. The magnetic fields used for imaging can align only a few of every
million magnetic nuclei present. However, this is sufficient to produce a useful
NMR effect.
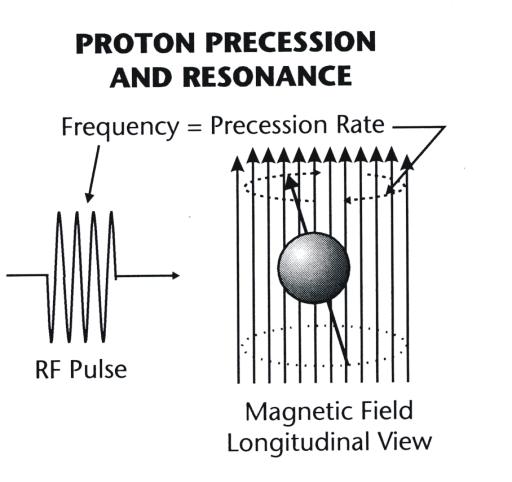
When a
spinning magnetic nucleus aligns with a magnetic field, it is not fixed; the
nuclear magnetic moment precesses, or oscillates, about the axis of the magnetic
field, as shown in Figure 3-3. The precessing motion is a physical phenomenon
that results from an interaction between the magnetic field and the spinning
momentum of the nucleus.
|
Figure 3-3. Magnetic nuclei precession and
resonance in a magnetic field. |
 |
Precession is often observed with a child’s spinning top. A spinning top
does not stand vertical for long, but begins to wobble, or precess. In this
case, the precession is caused by an interaction between the earth’s
gravitational field and the spinning momentum of the top.
The precession rate (cycles per second) is directly proportional to the
strength of the magnetic field. It is this precessing motion that makes a
nucleus sensitive and receptive to incoming RF energy when the RF frequency
matches the precession rate. This precession rate corresponds to the resonant
frequency. It is the precessing nuclei, typically protons, that are tuned to
receive and transmit RF energy.
|
Figure 3-4. The excitation of a magnetic nucleus by the
application of a pulse of RF energy. |
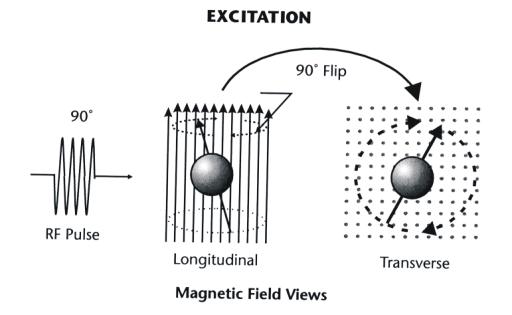 |
In MRI an RF pulse is used that flips some of the nuclei into the
transverse plane of the magnetic field. In this excited state the precession is
now transformed into a spinning motion of the nucleus around the axis of the
magnetic field. It should be noted that this spinning motion is an enhanced
precession and is different from the intrinsic spin of a nucleus about its own
axis.
The significance of a magnetic nucleus spinning around the axis of the
magnetic field is that this motion now generates an RF signal as shown in Figure
3-5. It is this signal, from many nuclei, that is collected to form the MR
image.
|
Figure 3-5. RF signal production by
magnetic nuclei spinning |
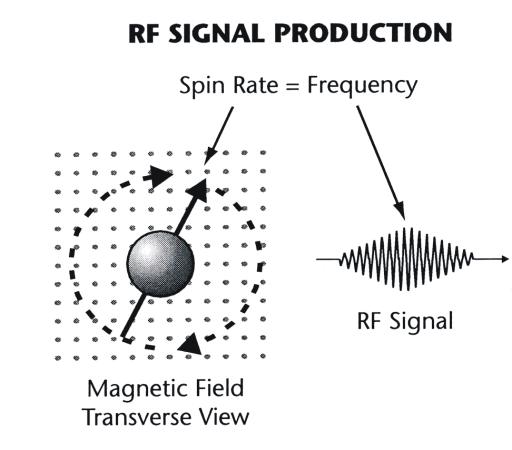 |
Relaxation is not instantaneous following an excitation. It cannot occur
until the nucleus is able to transfer its excess energy. How quickly the energy
transfer takes place depends on the physical characteristics of the tissue. In
fact, the nuclear relaxation rate (or time) is, in many cases, the most
significant factor in producing contrast among different types of tissue in an
image.
We are
more interested in the collective relaxation of many nuclei that produce the
magnetization of tissue and will return to this point in the next chapter.
The
significance of the nuclear precession is that it causes the nucleus to be
extremely sensitive, or tuned, to RF energy that has a frequency identical with
the precession frequency (rate). This condition is known as resonance and
is the basis for all MR procedures. NMR is the process in which a nucleus
resonates, or “tunes in,” when it is in a magnetic field.
Resonance is fundamental to the absorption and emission of energy by many
objects and devices. Objects are most effective in exchanging energy at their
own resonant frequency. The resonance of an object or device is determined by
certain physical characteristics. Let us consider two common examples.
Radio receivers operate on the principle of resonant frequency. A
receiver can select a specific broadcast station because each station transmits
a different frequency. Tuning a radio is actually adjusting its resonant
frequency. Its receiver is very sensitive to radio signals at its resonant
frequency and insensitive to all other frequencies.
The strings of a musical instrument also have specific resonant
frequencies. This is the frequency at which the string vibrates to produce a
specific audio frequency, or musical note. The resonant frequency of a string
depends on the amount of tension. It can be changed, or tuned, by changing the
tension. This is somewhat analogous to the resonant frequency of a magnetic
nucleus being dependent on the strength of the magnetic field in which it is
located.
The
resonant frequency of a nucleus is determined by a combination of nuclear
characteristics and the strength of the magnetic field. The resonant frequency
is also known as the Larmor frequency. The specific relationship between
resonant frequency and field strength is an inherent characteristic of each
nuclide and is generally designated the gyromagnetic ratio. The Larmor
frequencies [in megahertz (MHz)] for selected nuclides in a magnetic field of 1
T are shown in Table 3-2.
Table 3-2. Larmor Frequencies for Selected Nuclides in a Magnetic Field
|
Nuclide |
Larmor Frequency
(MHz/T) |
|
Hydrogen-1
Fluorine-19
Phosphorous-31
Sodium-23
|
42.58
40.05
17.24
11.26
|
When a proton, or other magnetic nucleus, is part of a molecule, it is
slightly shielded from the large magnetic field. The amount of shielding depends
on the chemical composition of the molecule. This means that protons in
different chemical compounds will be in slightly different field strengths and
will therefore resonate at different frequencies. This change in resonant
frequency from one compound to another is known as chemical shift. It can
be used to perform chemical analysis in the technique of MR spectroscopy and to
produce images based on chemical composition. However, in conventional MRI the
chemical-shift effect can be the source of an unwanted artifact.
In tissue the chemical shift in resonant frequency between the fat and
water is approximately 3.3 ppm, as shown in Figure 3-6. At a field strength of
1.5 T the protons have a basic resonant frequency of approximately 64 MHz.
Multiplying this by 3.3 gives a water-fat chemical shift of approximately 210
Hz. At a field strength of 0.5 T the chemical shift would be only 70 Hz.
|
Figure 3-6. The chemical shift effect on
the relative resonant frequency |
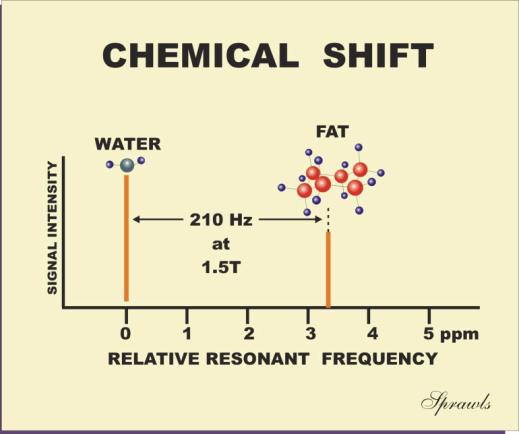 |
There are several imaging techniques that can be used to selectively
image either the water or fat tissue components. One approach is to suppress
either the fat or water signal with specially designed RF pulses. This technique
is known as spectral presaturation and will be described in Chapter 8.
Another technique makes use of the fact that the signals from water and fat
are not always in step, or in phase, with each other and can be separated to
create either water or fat images.
Nuclear Magnetic Resonance
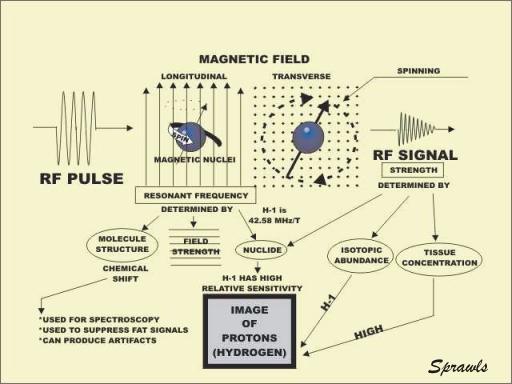
When a magnetic nucleus is located in a strong magnetic field, it
resonates. In effect, it becomes a tuned radio receiver and transmitter. The
resonance occurs because the spinning nucleus precesses at a rate that is in the
radio frequency range. The resonant frequency is determined by three factors.
Each specific nuclide has a unique resonant frequency. The resonant frequency is
affected to a small degree by the structure of the molecule containing the
magnetic nucleus. This, the chemical shift effect, is useful for spectroscopy
and to suppress fat signals in images. It can also lead to a certain type of
image artifact. The resonant frequency is directly proportional to the strength
of the magnetic field. This is useful because it makes it possible to tune the
various parts of a body to different frequencies by applying magnetic field
gradients.
When an RF pulse is applied to a magnetic nucleus oriented in the
longitudinal direction, it can be flipped into the transverse plane. There the
nucleus spins around the axis of the magnetic field and generates an RF signal.
It is the signals from many spinning nuclei that are collected and used to form
the image. It is necessary to have strong signals to produce good images. Signal
strength depends on three factors. Each magnetic nuclide has a unique
sensitivity or relative signal strength. All chemical elements have several
different isotopes, but all isotopes of an element are usually not in the form
of magnetic nuclei. Therefore, the abundance of the magnetic isotope for a
specific element has a major effect on signal strength. To produce strong
signals a tissue must have a relatively high concentration of a chemical element
and the most abundant isotope of that element must be magnetic.
Hydrogen is the only chemical element with a high concentration in tissue
and body fluids in the form of an isotope that has a magnetic nucleus.
Therefore, MR imaging is essentially limited to visualizing only one chemical
element, hydrogen.